Types of Glioblastoma: Subtypes, Prognosis & Treatment Explained
Glioblastoma is one of the most aggressive and complex types of brain cancer, characterized by rapid growth, resistance to treatment, and a poor outlook. This grade IV astrocytoma, often called glioblastoma multiforme (GBM), originates from glial cells in the brain and, despite advances in neurosurgery, radiation, and chemotherapy, remains a major clinical challenge.
To enhance patient survival and develop targeted therapies, it is crucial to understand the different types of glioblastomas, including their clinical behavior and genetic mutations. This article examines the main glioblastoma subtypes, along with their molecular features and implications for prognosis and personalized treatment.

What Is Glioblastoma?
Glioblastoma is a malignant primary brain tumor that arises from astrocytes, which are star-shaped glial cells that support neurons. GBMs can be identified by
- Highly infiltrative growth
- Rapid cell division
- New blood vessel formation, or angiogenesis
- Necrosis, or tumor-related cell death
- Resistance to conventional therapies
While complete resection is rare, modern neurosurgery typically aims for maximal safe resection, which is associated with better survival rates. Despite aggressive treatment, most glioblastomas recur within months.
Glioblastoma: Primary versus Secondary
Based on their place of origin, glioblastomas are generally divided into two clinical types:
1. Primary Glioblastoma (de novo)
Primary glioblastomas occur suddenly and without progression from lower-grade tumors. They are most common in older adults (median age ~64). They are typically faster growing and more aggressive, with IDH-wildtype, EGFR amplification, PTEN loss, and TERT promoter mutations, resulting in a poorer prognosis than secondary GBMs.
2. Secondary Glioblastoma
It develops from lower-grade gliomas, such as diffuse or anaplastic astrocytomas, and usually affects young people. They progress slowly at first but eventually develop into aggressive grade IV tumors. These glioblastomas frequently contain IDH1/IDH2 mutations, TP53 mutations, and ATRX loss—genetic markers linked to a slightly better prognosis than primary glioblastomas.
Knowing whether a tumor is primary or secondary can influence treatment and prognosis.
Molecular Subtypes of Glioblastoma
Thanks to advances in genomic profiling, it is now known that glioblastoma is a disease with multiple molecular subtypes. Every subtype exhibits unique genetic changes, biological behaviors, and therapeutic responses.

1. IDH-Mutant Glioblastoma: This subtype features mutations in the IDH1 or IDH2 genes and is more common in secondary glioblastomas and younger patients. IDH mutations produce the oncometabolite 2-HG, which disrupts normal cell regulation. These tumors are typically less aggressive and offer a better prognosis with improved response to therapy.
Treatment Approach: May include standard therapies with consideration for clinical trials targeting IDH pathways.
2. IDH-Wildtype Glioblastoma: This subtype, which primarily affects older adults, makes up 90% of all glioblastoma cases that do not have IDH mutations. It is usually associated with a poor prognosis and is more aggressive. Chromosome 10 loss, TERT promoter mutations, and EGFR amplification are examples of common genetic changes.
MGMT Promoter Methylation as a Predictive Biomarker.
The MGMT (O6-methylguanine-DNA methyltransferase) promoter’s methylation status is another important molecular marker in glioblastoma.
- MGMT Function: Repairs alkylated DNA damage caused by chemotherapy drugs such as temozolomide.
- Methylated MGMT Promoter.
- MGMT expression is silenced.
- Temozolomide has a greater effect on tumors.
- Linked with improved survival.
- Unmethylated MGMT Promoter:
- Active DNA repair.
- Less responsive to chemotherapy.
➤ Clinical Use: Testing for MGMT promoter methylation status is now standard practice in newly diagnosed glioblastoma to guide treatment decisions.
Genetic and Molecular Profiling in Glioblastoma
Modern cancer care is increasingly based on molecular diagnostics to guide personalized treatment plans. The following genetic changes are frequently examined in glioblastoma: These changes not only help to stratify prognosis, but they also open up the possibility of targeted therapies.
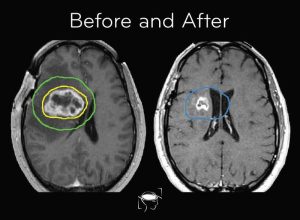
Standard Treatment Options For Glioblastoma
The standard of care for glioblastoma is essentially the same despite molecular diversity:
- Surgery: The safest way to remove a tumor is through surgery. Despite not being a cure, it improves survival and reduces the mass effect.
- Radiation therapy: It is given for six weeks following surgery. Treats any microscopic disease that may still exist.
- Chemotherapy: The most common chemotherapy used as an adjuvant treatment and in conjunction with radiotherapy is temozolomide (TMZ). Works better in patients with MGMT-methylated tumors. Accordingly, speak with your provider.
Tumor-Treating Fields (TTFields): A non-invasive treatment that stops cancer cells from proliferating by applying electric fields. Temozolomide is used as a maintenance adjunct.
Targeted Therapies and Future Approaches
Emerging therapies aim to exploit glioblastoma’s molecular weaknesses. Some promising strategies include
- IDH Inhibitors (e.g., ivosidenib): For IDH-mutant GBMs.
- EGFR Inhibitors: Limited, still under investigation (due to drug penetration and the presence of multiple resistance pathways).
Immunotherapy:
- Checkpoint inhibitors (e.g., PD-1 blockers) have shown limited success in unselected GBM patients; research is ongoing to identify specific patient populations or combinations that may benefit more.
- Cancer vaccines and CAR T-cell therapies are under clinical trials.
- Gene therapy and oncolytic viruses are engineered viruses that selectively infect and kill tumor cells.
Precision Medicine:
Using a patient’s tumor molecular profile to match targeted drugs represents the future of glioblastoma treatment.
Prognosis Based on Subtype
Glioblastoma prognosis remains poor, with a median survival of 12–15 months despite aggressive treatment. However, prognosis can vary:
| Subtype | Median Survival |
| IDH-Mutant GBM | 2–4 years |
| IDH-Wildtype GBM | 12–15 months |
| MGMT Methylated | Up to 18–20 months |
| MGMT Unmethylated | 10–12 months |
Long-term survivors do exist, especially among younger patients carrying favorable genetic markers.
Monitoring & Recurrence
Even after aggressive treatment, recurrence is almost inevitable in glioblastoma. Regular follow-ups include
- MRI scans every 2–3 months
- Neurocognitive evaluations
- Monitoring for treatment-related toxicity
Treatment options for recurrence include
- Re-surgery (if possible)
- Re-radiation
- Clinical trials
- Bevacizumab (anti-VEGF antibody)
- Experimental therapies
Final Thoughts
Although glioblastoma is a diverse and deadly brain cancer, there is hope thanks to advancements in genomic knowledge, biomarker identification, and targeted treatments. Predicting results and guiding individualized treatment depend on precise classification into IDH-mutant vs. wildtype, MGMT methylation status, and molecular changes.
FAQs
What is the difference between glioblastoma and glioma?
Glioblastoma is the most aggressive type of glioma. Gliomas are a broader category of brain tumors arising from glial cells.
Can glioblastoma be cured?
Currently, there is no cure for glioblastoma. Treatments aim to prolong survival and improve quality of life.
How is glioblastoma diagnosed?
Through MRI imaging and confirmed by surgical biopsy, followed by histopathology and molecular testing.
Does everyone with glioblastoma get genetic testing?
Yes, testing for IDH status and MGMT methylation is standard. Additional tests may be ordered for personalized therapy.